

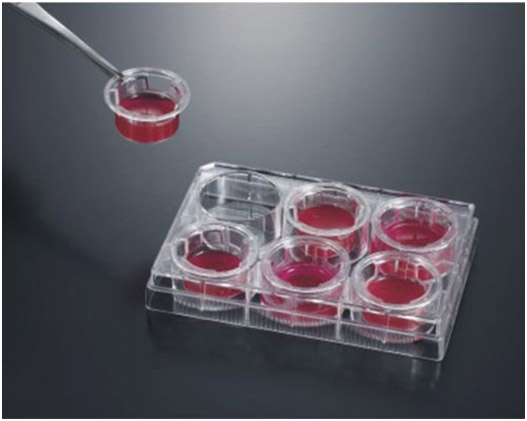
The polycarbonate plate inserts feature a thin, semitransparent polycarbonate membrane that is available in six pore sizes from 0.1μm to 12.0μm. All inserts are treated for optimal cell attachment. The polycarbonate plate inserts are sterile and assembled with well plates and resist most fixing and staining agents. All plates come with lids.
Tissue Culture Plate Inserts feature a thin, microscopically transparent polyester membrane that is tissue culture treated for optimal cell attachment and growth. Tissue Culture Plate Inserts provide excellent cell visibility under phase contrast microscopy and allow assessment of cell viability and monolayer formation. Tissue Culture Plate Inserts are sterile and can be assembled with 6, 12 and 24 well plates. All plates come with lids.

| Polycarbonate Membrane | Polyester Membrane | |||||
|---|---|---|---|---|---|---|
| Cell Culture insert-6 well |
Cell Culture insert-12 well |
Cell Culture insert-24 well |
Cell Culture insert-6 well |
Cell Culture insert-12 well |
Cell Culture insert-24 well |
|
| Material | Virgin Gamma resistant polycarbonate | Virgin Gamma resistant polycarbonate | Virgin Gamma resistant polycarbonate | Virgin Gamma resistant polyester | Virgin Gamma resistant polyester | Virgin Gamma resistant polyester |
| Insert Diameter(mm) | 24 | 12 | 6.5 | 24 | 12 | 6.5 |
| Growth Area(cm²) | 4.67 | 1.12 | 0.33 | 4.67 | 1.12 | 0.33 |
| Volume Added Per Plate(ml) | 2.6 | 1.5 | 0.6 | 2.6 | 1.5 | 0.6 |
| Volume Added To Inside of Plate Insert(ml) | 1.5 | 0.5 | 0.1 | 1.5 | 0.5 | 0.1 |
| Packing | 6/box, 24/case | 12/box, 48/case | 12/box, 48/case | 6/box, 24/case | 12/box, 48/case | 12/box, 48/case |
| Purity grade(s) | Sterile, pyrogen-, DNase-, RNase-, human and bacterial DNA-free. Non-cytotoxic | Sterile, pyrogen-, DNase-, RNase-, human and bacterial DNA-free. Non-cytotoxic | Sterile, pyrogen-, DNase-, RNase-, human and bacterial DNA-free. Non-cytotoxic | Sterile, pyrogen-, DNase-, RNase-, human and bacterial DNA-free. Non-cytotoxic | Sterile, pyrogen-, DNase-, RNase-, human and bacterial DNA-free. Non-cytotoxic | Sterile, pyrogen-, DNase-, RNase-, human and bacterial DNA-free. Non-cytotoxic |
| Sterilization | 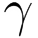 - irradiation - irradiation |
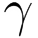 - irradiation - irradiation |
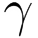 - irradiation - irradiation |
 - irradiation - irradiation |
 - irradiation - irradiation |
 - irradiation - irradiation |
| Cat. No. | Well | Pore size(μm) | Growth area for insert membrane*(cm2) |
Sterile | Qty. per box/case |
|---|---|---|---|---|---|
| TCS000006 | 6 | 0.1 | 4.67 | Y | 6/24 |
| TCS001006 | 6 | 0.4 | 4.67 | Y | 6/24 |
| TCS005006 | 6 | 1.0 | 4.67 | Y | 6/24 |
| TCS002006 | 6 | 3.0 | 4.67 | Y | 6/24 |
| TCS003006 | 6 | 8.0 | 4.67 | Y | 6/24 |
| TCS100006 | 6 | 12.0 | 4.67 | Y | 6/24 |
| TCS000012 | 12 | 0.1 | 1.12 | Y | 12/48 |
| TCS001012 | 12 | 0.4 | 1.12 | Y | 12/48 |
| TCS005012 | 12 | 1.0 | 1.12 | Y | 12/48 |
| TCS002012 | 12 | 3.0 | 1.12 | Y | 12/48 |
| TCS003012 | 12 | 8.0 | 1.12 | Y | 12/48 |
| TCS100012 | 12 | 12.0 | 1.12 | Y | 12/48 |
| TCS000024 | 24 | 0.1 | 0.33 | Y | 12/48 |
| TCS001024 | 24 | 0.4 | 0.33 | Y | 12/48 |
| TCS005024 | 24 | 1.0 | 0.33 | Y | 12/48 |
| TCS002024 | 24 | 3.0 | 0.33 | Y | 12/48 |
| TCS003024 | 24 | 8.0 | 0.33 | Y | 12/48 |
| TCS100024 | 24 | 12.0 | 0.33 | Y | 12/48 |
| TCS004024 | 24 | 5.0 | 0.33 | Y | 12/48 |
| Cat. No. | Well | Pore size(μm) | Growth area for insert membrane*(cm2) |
Sterile | Qty. per box/case |
|---|---|---|---|---|---|
| TCS010006 | 6 | 0.1 | 4.67 | Y | 6/24 |
| TCS011006 | 6 | 0.4 | 4.67 | Y | 6/24 |
| TCS015006 | 6 | 1.0 | 4.67 | Y | 6/24 |
| TCS012006 | 6 | 3.0 | 4.67 | Y | 6/24 |
| TCS013006 | 6 | 8.0 | 4.67 | Y | 6/24 |
| TCS010012 | 12 | 0.1 | 1.12 | Y | 12/48 |
| TCS011012 | 12 | 0.4 | 1.12 | Y | 12/48 |
| TCS015012 | 12 | 1.0 | 1.12 | Y | 12/48 |
| TCS012012 | 12 | 3.0 | 1.12 | Y | 12/48 |
| TCS013012 | 12 | 8.0 | 1.12 | Y | 12/48 |
| TCS010024 | 24 | 0.1 | 0.33 | Y | 12/48 |
| TCS011024 | 24 | 0.4 | 0.33 | Y | 12/48 |
| TCS015024 | 24 | 1.0 | 0.33 | Y | 12/48 |
| TCS012024 | 24 | 3.0 | 0.33 | Y | 12/48 |
| TCS013024 | 24 | 8.0 | 0.33 | Y | 12/48 |
The cell culture inserts are manufactured in accordance with ISO13485:2003 & ISO9001:2008 quality management systems in class 100,000 clean room. Radiation protocols, Quality certificate and Certificate of Compliance are provided with every shipment upon request

| Component Material | 1. Virgin Gamma resistant Polycarbonate meets USP Class VI requirements. 2. Virgin Gamma resistant Polyester meets USP Class VI requirements |
| Sterilization Assurance Level | SAL 10 ‾6. Every lot has been irradiated and dosimetrically released. Procedures for determining the dosage comply with ISO11137 |
| Non-Pyrogenic | Products are validated to have less than 0.5EU/ml |
| Cytotoxicity | All material resins are qualified using USP and /or ISO 10993 standards for cytotoxicity and have been shown to be non-toxic |
| DNase & RNase Free | Products are labeled DNase & RNase free and have been validated |
| Quality Control Testing | Representative samples have been selected from each lot and inspected according to ZK-QI |
| Tissue Culture testing | Treated samples are randomly taken from a total lot for actual perfomance test for growth with Hep-g2( Human Hepatic carcinoma cells) |
The cell culture inserts are placed inside appropriate well plate and each plate is individually packed. Every case is printed with lot No. for quality traceability as well as expiry date. We warrant that the packaging is sufficient to protect the products against any damage during transit and storage.

Cell and Tissue culture products series are all innovatively designed by JET Engineers and manufactured under the control of ISO 9001: 2008 and ISO 13485 quality management systems. All the JET Biofil product are manufactured with 100% USP VI crystal class virgin polystyrene ( GPPS) and equal high quality polyethylene ( PE) to eliminate all extraneous materials and ensure the integrity. Furthermore, the high transparency of high class material ensures good observability. In addition, all the products are sterilized by gamma irradiation, certified DNase RNase free and non- Pyrogenic. Products are modified with vacuum gas plasma, causing the very hydrophobic polystyrene surface to become negatively charged and hydrophilic, allowing the cells to attach evenly and consistently.

In addition to this, BioStar Lifetech Offers a Complete Satisfaction Guarantee So You Can Be Confident of Your Purchasing Decision. If, For Any Reason You Are Not Satisfied With The Product Performance Or Service Provided, We Will Either Replace Or Issue A Refund For The Purchase Price Of Your Product.
